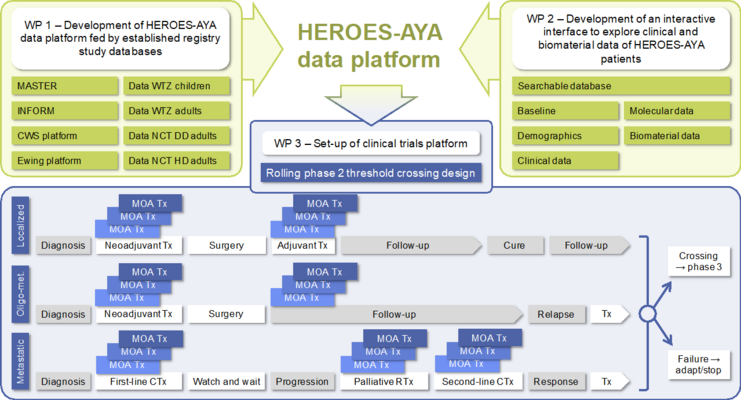
Subproject 6: Clinical implementation
Implementation of the Rolling-2 study design in the HEROES-AYA registry platform
Subproject 6 consists of four major work packages.
-
Creation of a common data platform, which is fed by several register/intervention study databases. (INFORM, MASTER, the SoTiSaR registry of the CWS, the international EuroEwing registry and the EuroEwing Phase 3 study of the Cooperative Ewing Sarcoma Study Group [CESS], SarcBOP and the cancer registries in Dresden and Essen);
-
Provision of an overview of HEROES-AYA patients for subprojects 1-5, providing clinical data and an overview of which biomaterials are available, including clinical and biomaterial information;
-
Development of new clinical studies from the results of subprojects 1-5. The clinical studies are designed according to an innovative study design, the so-called Rolling-2-Design. This enables studies to be set up and conducted in a short period of time, which is very advantageous for research into new drugs for rare diseases.
-
Creation of a harmonized database and platform.
Main objectives:
-
Implementation of the integrative data platform for HEROES-AYA
-
Provision of an overview of HEROES-AYA patients, including clinical data and available biomaterials
-
Development of innovative interventional studies with special consideration of the AYA patient population